Car Airbag Chemical Reaction
Up to 24 cash back 2NaN3 2Na 3N2 10Na2KNO3 K2O5NaN K2O Na2O SiO2 Na2K2SiO4. Chemical Reactions Assignment ByTiffany Hogg Importance of the chemical reaction Along with wearing a seat belt car air bags saved the lives of about 26 of people in frontal collisions and 32 of people not wearing a seatbelt during the accident.

Kings Chemistry Chemistry Of Airbags In Cars Kings Facebook
It creates an electric current that triggers a heat source in the airbag canister.
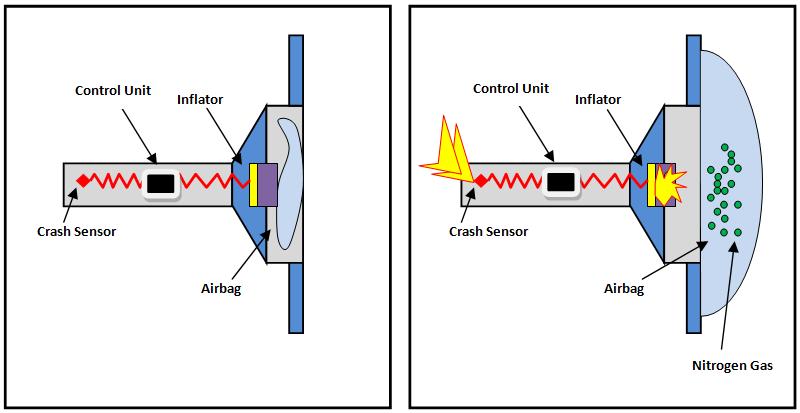
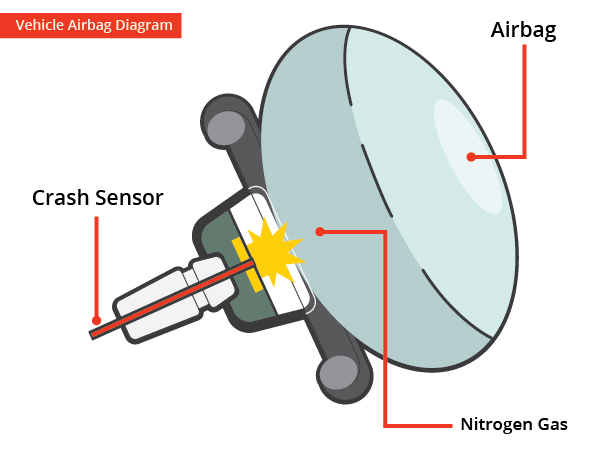
. The airbags inflation system reacts sodium azide NaN3 with potassium nitrate KNO3 to produce nitrogen gas. A car engine uses this reaction to push its pistons. When car sensors detect a crash a chemical reaction is triggered by the ignition of a solid hunk of propellant.
NaN3 s -- Na s N2 g Reaction 2. In fact it is so reactive it can light up when in touch with water. This shows just how fast the chemical reactions occur inside an airbag.
Hot blasts of the nitrogen inflate the airbag. When this happens the airbag inflates without warning and shoots out metal fragments toward the driver and passenger. Over time when exposed to variations in high air temperature and moisture fluctuations ammonium nitrate can become unstable acting as a propellant.
Airbags deploy upon impact to minimize serious injuries to passengers. Front Passenger Airbags In the event of a collision the passenger airbag will deploy from the dashboard of the car. CRASHES trip sensors in cars that send an electric signal to an ignitor.
The airbags inflation system reacts sodium azide NaN3 with potassium nitrate KNO3 to produce nitrogen gas. Sodium Azide is stable at room conditions however when heated it breaks down. The chemical reaction produces a gas that inflates the airbag the gas that the chemical reaction produces is nitrogen gas.
The reactions that occur inside a standard automobile airbag. Sodium Azide Potassium Nitrate Silicon Dioxide are the initial reactants packed into the air bag module. This requirement is satisfied in many automotive airbag systems through use of explosive chemical reactions one common choice being the decomposition of sodium azide NaN 3.
Up to 24 cash back Most airbags inflate in 130th of a second and travel at around 200 mph. If youre in a car accident you want to be sure your airbags protect you. Inside the engine the gas is sprayed into the combustion chamber above the piston.
Chemical Reaction and a matter of few milliseconds. But the other product of the reaction sodium is not a benign chemical. And they work because of chemistry with some physics thrown in.
All of these difficulties took 30 years for airbags to be commonly available in the year 1980s. The reaction occurs when the car s sensors detect that a crash has occurred. Something that I noticed in my research about airbags is that they are structured much like a shotgun shell.
Chemical Reaction Behind AirbagsDESCRIPTIONAccording to Association for Safe International Road Travel nearly 13 million people die in road crashes. The chemical inside the airbag that is responsible for the spontaneous reaction is called Sodium Azide- NaN3. Those reactions are listed above.
Reactions Science Videos May 01 2018. Air bags have been saved lives and have. These issues were addressed in the 1970s with the invention of small propellant inflators devices that initiate a chemical reaction that releases hot nitrogen gas into the airbag.
CHEMICAL REACTION IN CAR AIRBAGS by hamza mumtaz. Passanger air bags reduced 14. The way propellant works in.
Airbags are meant to work in conjunction with seatbelts so buckle up. This week on Reactions were talking the science of airbags. When an airbag goes of an impact makes an accelerometer create a spark the.
The airbag and inflation system stored in the steering wheel. Explore how decomposition reactions are used to inflate an airbag in a car crash. 338 moles of sodium azide must be packed into the air bag module.
The heat generated causes sodium azide to decompose into sodium metal and nitrogen gas which inflates the cars air bags. The reaction -- basically a little explosion -- heats it all up making the air expand and push the piston outward. When sensors in the vehicle detect a collision an electrical current is passed through a.
This device was a major stepping-stone in the development of airbag technology as it has enabled the common use of commercial airbag systems that have been available since the late. See more car safety images. What is the chemical reaction in airbags.
The science behind the inflation of an airbag is that the airbag is inflated when it successfully goes through a chemical reaction. This whole process has to happen really fastwithin about 30 milliseconds 003 secondsin order to cushion the passengers in the car against impact. There are three reactions involved in the deployment of an air bag.
Air bags is used as a protective device for the users of cars. Na s KNO3 s -- K2O s Na2O s N2 g. The culprit of the airbag defect is a compound called ammonium nitrate.
Hot blasts of the nitrogen inflate the airbag. TABLE OF REACTIONS GAS GENERATOR REACTANTS PRODUCTS REACTION INITIAL REACTION TRIGGERED NaN3 Na BY SENSOR N2g SECOND REACTION Na K2O KNO3 NA2O N2 g FINAL REACTION K2O Alkaline Na2O Silicate SiO2 glass ADVANTAGE OF CAR AIR BAG. When the steering wheel airbag module receives a deployment signal the igniter switch triggers a chemical reaction which causes the airbag to inflate instantly.
This heat causes the chemicals in the airbag to react inflating the airbag. Based on the chemical equation 2 NaN 3 -- 2 Na 3 N 2 a cup of the compound can easily produce enough nitrogen gas to fill a standard airbag which is close to 70 liters. It is mixed with oxygen and then ignited.

Clemson Vehicular Electronics Laboratory Airbag Inflators
Comments
Post a Comment